Abstract

Histiocytic neoplasms are clonal disorders of the monocyte/macrophage lineage that include Langerhans cell histiocytosis (LCH) and Erdheim-Chester disease (ECD). Histiocytoses are defined by recurrent, mutually exclusive mutations activating MAP kinase signaling, including BRAFV600E mutations in ~50% of LCH and ECD patients, which confer robust lasting clinical responses to BRAF inhibition. Mutations in MEK1/2, ARAF, and a diverse array of other kinases are present in BRAF wild-type histiocytoses patients and are broadly responsive to the allosteric MEK1/2 inhibitor cobimetinib.
Following genomic analysis of ~300 pediatric and adult patients with diverse histiocytoses, the single most common genomic alteration following BRAFV600E mutations are MEK1 E102_I103 in-frame deletions. In contrast to BRAFV600E, the biological impact of activating mutations in MEK1/2 kinases are unknown, and no cancer associated MEK1/2 mutation has ever been modeled in vivo. MEK1/2 mutations are recurrent across a wide variety of human cancers and RASopathies and are now often found at resistance to emerging KRAS-mutant selective inhibitors. These findings motivated us to generate Map2k1E102_I103del conditional knock-in mice within the endogenous locus of Map2k1 (encoding MEK1 kinase), which represent the first genetically engineered mouse model of MEK1/2 mutations.
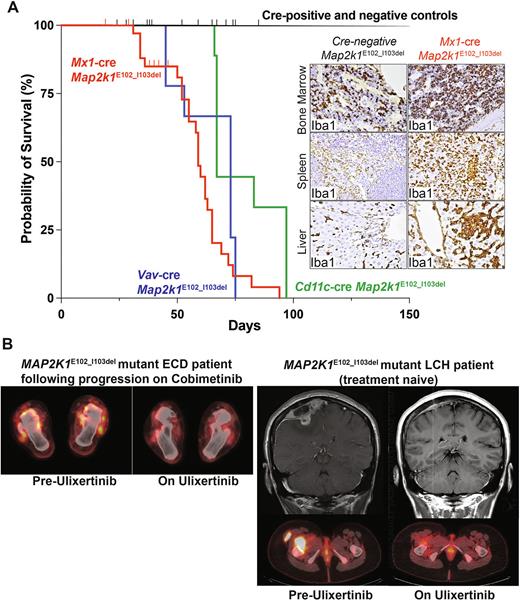
We first crossed Map2k1E102_I103del mice to inducible Mx1-cre driver mice. Interestingly, Mx1-cre Map2k1E102_I103del knock-in mice developed a 100% penetrant lethal myelomonocytic disorder by 5-6 weeks of age while their cre-negative Map2k1E102_I103del littermates did not develop disease (Figure A). Notably, recombination of the Map2k1E102_I103del alleleoccurred spontaneously without pIpC administration and resulted in strong activation of phosphorylated ERK1/2 in bone marrow and spleen mononuclear cells. Mx1-cre Map2k1E102_I103del mice developed a myelomonocytic neoplasm with suppression of B-lymphopoiesis and expansion of classical and non-classical monocyte subsets, macrophages, and Cd11b+ conventional dendritic cells. Monocyte, macrophage, and dendritic cells infiltrated hematopoietic organs (bone marrow, spleen, and peripheral blood), as well as liver and skin, each of which are common sites of LCH and ECD disease involvement.
The Mx1-cre Map2k1E102_I103del disease was hematopoietic cell autonomous and could be propagated by serial hematopoietic reconstitution in irradiated recipient mice, as well as using Vav1-cre Map2k1E102_I103del knock-in mice (where Cre is expressed only in hematopoietic cells from earliest hematopoietic development). Finally, restricting MEK1 mutant expression to monocyte and dendritic cell precursors using CD11c-cre resulted in similar lethal expansions of monocyte, macrophage, and dendritic cells as seen in the Mx1-cre and Vav1-cre models.
Prior in vitro work has demonstrated that MEK1 p.E102_I103del activates MEK and ERK without requiring activated GTP-bound RAS and is associated with resistance to allosteric MEK inhibitors. Consistent with this, we identified nine histiocytosis patients with this specific MEK1 mutation, three of which had de novo resistance to cobimetinib. These three patients and one treatment-naïve patient were treated with an oral ERK1/2 inhibitor ulixertinib under the FDA single-patient compassionate use program. Three of 4 patients had a clinical or radiological partial or complete response to ulixertinib (Figure B).
Overall, these data identify the impact of oncogenic MEK kinase mutations in vivo as found in children and adults with histiocytoses and RASopathies. Expression of a RAF-independent MEK1 mutation in vivo gave rise to disease reminiscent of human systemic histiocytic neoplasms. Importantly, this molecular entity is resistant to MEK inhibition in some patients, a phenomenon previously undocumented, but responsive to ERK inhibition, which may be a promising therapeutic approach to histiocytic neoplasms unresponsive to allosteric MEK inhibition. The clinical observation of efficacy of ERK inhibition in MEK inhibitor resistant histiocytoses patients has resulted in a soon-to-be initiated focused phase 2 clinical trial of ulixertinib in adults with histiocytoses.
Disclosures
Abdel-Wahab:H3B Biomedicine, Foundation Medicine Inc, Merck, Prelude Therapeutics, and Janssen: Consultancy; Envisagenics Inc., AIChemy, Harmonic Discovery Inc., and Pfizer Boulder: Membership on an entity's Board of Directors or advisory committees; H3B Biomedicine, LOXO Oncology, and Nurix Therapeutics: Research Funding. Diamond:Day One Biopharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Springworks Therapeutics: Membership on an entity's Board of Directors or advisory committees; Pfizer: Other: unpaid editorial support.
OffLabel Disclosure:
The allosteric MEK1/2 inhibitor cobimetinib and an ERK1/2 inhibitor ulixertinib are being used in an in vivo conditional knock-in mouse model of a myeloproliferative myelomonocytic neoplasm reminiscent of a histiocytic neoplasm. Also, there is use of ulixertinib (an ERK1/2 inhibitor) in few patients with histiocytic neoplasms under the FDA single-patient compassionate use program.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal